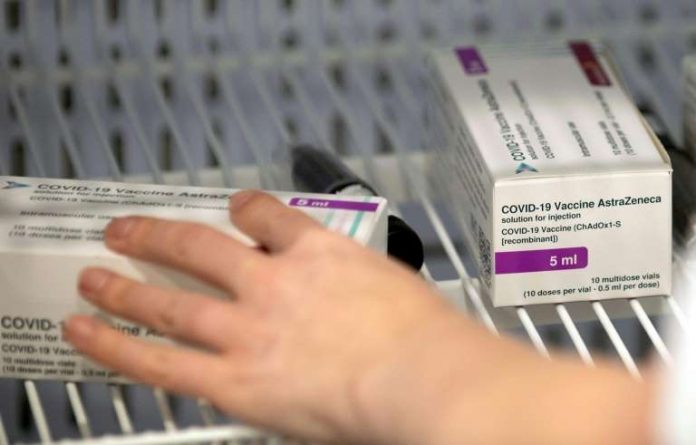
WHO on Monday gave the green light to two versions of the AstraZeneca/Oxford Covid-19 vaccine for emergency use, allowing them to be rolled out globally via Covax. – File photo
GENEVA (Feb 16): The World Health Organisation (WHO) on Monday gave the green light to two versions of the AstraZeneca/Oxford Covid-19 vaccine for emergency use, allowing them to be rolled out globally via Covax.
The two versions are produced by AstraZeneca-SKBio in South Korea and the Serum Institute of India, WHO said in a news release, noting that the vaccine shows 63.09 per cent efficacy and “is suitable for low- and middle-income countries due to easy storage requirements”, reports Xinhua news agency.
The vaccine can now be used via Covax, a WHO-led international initiative for equitable access to Covid-19 vaccines, said Dr Mariangela Simao, WHO assistant-director general for Access to Medicines and Health Products.
“Countries with no access to vaccines to date will finally be able to start vaccinating their health workers and populations at risk,” said Simao.
“But we must keep up the pressure to meet the needs of priority populations everywhere and facilitate global access,” she said. And to this end, she sorted out two priorities: a scale-up of manufacturing capacity, and developers’ early submission of their vaccines for WHO review.
The AstraZeneca product is the second Covid-19 jab to have received WHO authorisation, after Pfizer/BioNTech vaccine in late December. – Bernama
The post WHO approves AstraZeneca Covid-19 vaccine for emergency use appeared first on Borneo Post Online.