Subscribe to our Telegram channel for the latest updates on news you need to know.
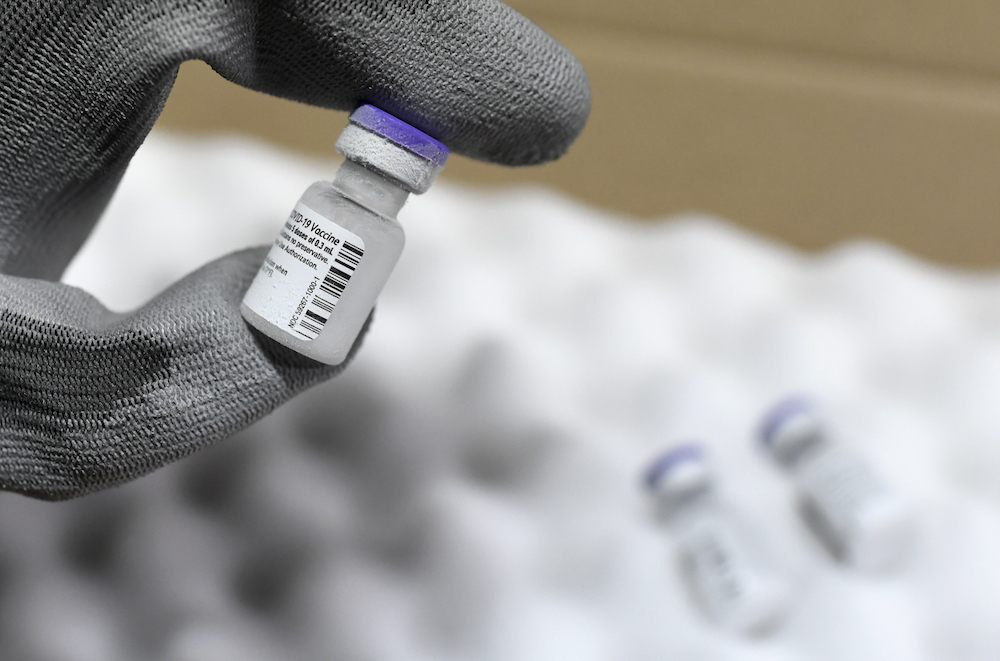
KUALA LUMPUR, Feb 16 — There is no short cut for Covid-19 vaccines to enter the Malaysian market as they are subject to strict conditions to ensure they are safe, effective and of quality.
Malaysian Pharmacists Society (MPS) president Amrahi Buang said Covid-19 vaccines that are registered in other countries must be approved by the National Pharmaceutical Regulatory Agency (NPRA) to be brought into the country.
He said the vaccines have to go through a process which was required for other vaccines that had entered the Malaysian market.
“This means that the vaccine will go through the pre-clinical phases I, II, III and after it has been registered in Malaysia, it will enter the next phase.
“When there is a (new) vaccine in Malaysia, it must meet three main conditions, namely it is safe, effective and of quality.
“This is what we want Malaysians to know, so there is no need to worry,” he said in a Bernama TV programme Koresponden today.
Meanwhile, Prime Minister Tan Sri Muhyiddin Yassin today launched the National Covid-19 Immunisation Programme Handbook, during which he announced that the POVF-BioNTech Covid-19 vaccine is expected to arrive this Sunday, with the National Covid-19 Immunisation programme to be implemented in stages beginning this February 26.
On some quarters having skepticism about the Covid-19 vaccine, Amrahi said the matter had been explained by Minister in the Prime Minister’s Department (Religious Affairs) Senator Datuk Dr Zulkifli Mohamad Al-Bakri.
According to the minister, a special meeting of the Muzakarah Committee of the National Council for Islamic Religious Affairs last Dec 3 had decided that the use of the Covid-19 vaccine is permissible (harus) and obligatory (wajib) for groups which have been identified by the government.
Amrahi said the Pfizer-BioNTech vaccine did not contain ingredients from animal sources.
“Therefore, we can assume that this vaccine is a halal product. Likewise, the Sinovac vaccine developed by a Chinese pharmaceutical company … the vaccine has been used in Indonesia and was declared halal by the Indonesian Ulama Council after conducting a study to ensure that the vaccine does not contain prohibited substances,” he added. — Bernama